Yes the current draw is very linear until Curie. That was the point of the work done over a year ago. But is a plot of the brass temp linear as well? If so you needn't bother with a regression - you need only know room temp and time to Curie and do the simple interpolation. BUT, the problem is LR88 didn't measure case temp (coil temp but not case temp) ... The two guys above can fill in that blank.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Induction brass annealer redux
- Thread starter Gina1
- Start date
I have 171 amps readings for a period of 4.1 sec - more than enough IMO. The red curve is the temperature of the brass. The blue is the amps. The IR readings are on the Y axis. X axis is time. The amps increase is only in the first milliseconds (inrush current) . Then the curve is flat.You need to sample more frequently I suspect. As the case heats you should see increasing amps and then a sudden drop when the curie temp is hit. See the charts in my linked to post. Can you add temp labels to the right axis?
What intrigues me is the moment of a sharp increase of the temperature of the brass just before the amps start decreasing. Looking forward to possible interpretations.
Of interest is the period up to Curie temp. It took circa 13 seconds for LR88's annealer to get his case to this point when the amps dropped off sharply. (The x axis is in 10ths of seconds.) Unfortunately he wasn't able to record case temp. (Coil temp is irrelevant IMO.)

You're getting there in a fraction of that time. It's this period that you should expand into on your plot. In your plot amps are falling from outset. I assume your left y axis is amps. Can you add the temp labels to the right y axis? And plot more observations from t=0 until your 4th marked data point?
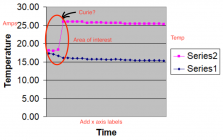
You're getting there in a fraction of that time. It's this period that you should expand into on your plot. In your plot amps are falling from outset. I assume your left y axis is amps. Can you add the temp labels to the right y axis? And plot more observations from t=0 until your 4th marked data point?

As I mentioned before - this the end of the process. From the beginning to that point the temperature and current curves are flat. I do not understand the relevance of your suggestion.
In addition, this is just a graphical/visual presentation:
I=f(time} and T=f(time) of the process.
In addition, this is just a graphical/visual presentation:
I=f(time} and T=f(time) of the process.
The messing with regression is a one time job, and I wrote the last sentence to specify I was not referring to the linear increase of current over time.Yes the current draw is very linear until Curie. That was the point of the work done over a year ago. But is a plot of the brass temp linear as well? If so you needn't bother with a regression - you need only know room temp and time to Curie and do the simple interpolation. BUT, the problem is LR88 didn't measure case temp (coil temp but not case temp) ... The two guys above can fill in that blank.
My observations were based on plotting current at time x and current at glow for different cases. Time to get to glow could be different between cases even though current at time x is the same for 2 different cases. Both would have linear increase of current over time, but not necessarily the same starting point and rate.
The curie point could be used as reference point, but you would have to destroy cases and find relationship between curie and the perfect anneal (along the lines of what AMP does).
I'm suggestiong again, another way along the lines of what the guys with IR sensors are doing - getting there based on current - knowing that if current at time x is 5 amps, then I would reach target at 7.1 (depending on the actual annealer - the messing part using linear regression, one time ever).
What the IR sensor guys (@oliverpsmile etc) could do is help confirming relationship between current and temperature. @oliverpsmile: Could you measure current at say 0.2 seconds and the point you measure 1000F for a few different cases? I suspect plotting those numbers (current at 0.2 on x, Current at 1000F on Y) would (hopefully) shows linearity - which would be useful for determining target using only current measurement.
Whether or not 1000F, 950F is the correct target for perfect anneal depends - and you could implement hold time translating time into energy which current and the already observed linear relationship between current and time for a specific case can be used for using interpolation.
LR88 observed that current was not flat - as one would expect. The resistance (load) of the brass in the work coil changes as it heats.As I mentioned before - this the end of the process. From the beginning to that point the temperature and current curves are flat. I do not understand the relevance of your suggestion.
In addition, this is just a graphical/visual presentation:
I=f(time} and T=f(time) of the process.
https://www.allaboutcircuits.com/textbook/direct-current/chpt-12/temperature-coefficient-resistance/
The temp coefficient of brass is 1.5 x 10^-3 per degree Celcius. Standardelg could see how this fits into his current regression with a slope of 1.64. Resistivity and temp coefficients (alpha) below.
| Brass - 58% Cu | 5.9 x 10-8 | 1.5 x 10-3 | |
| Brass - 63% Cu | 7.1 x 10-8 | 1.5 x 10-3 |
Current increases and increases in quite linear fashion until the brass hits its Curie temp and, all of a sudden, its properties change and the current draw drops sharply. He did not observe case temp. (Standardelg observed the same thing also.) So far no surprises. Of extreme interest is the rate of change of case temp throughout this period - from room temp to Curie. Given the increase in current draw is very linear one would expect the change in temperature of the brass to be rather linear as well (as it is its changing resistivity that's driving this change in current), but this requires 'proof'.
If it were a very linear progression it would be easy to estimate the time required for the case to reach a target temp by simple (linear) interpolation. If, however, it behaves in some other manner then the estimation is more complex. You are one of the very few people that can observe BOTH current draw and case temp.
I have no doubt that AMP examined measuring case temp - and likely with far greater resources than anyone here - and, we suspect, decided to build Aztec around monitoring current instead. Given we don't have access to Vickers hardness testing equipment and a pile of research, most of us have been working with 750F Tempilaq way down the case body - in my case seeking 750F one shoulder width below the body/shoulder junction - on the expectation that everything above hits a far higher temperature (and consistently so). You have improved upon this by observing case temp closer to the area of interest more directly (how accurate is your sensor?) and automating timing based on this. It would be great if you can help fill in some of the gaps with respect to the progression of case temp and current up to the point of Curie.
PM with the test data attachedCould you measure current at say 0.2 seconds and the point you measure 1000F for a few different cases? I suspect plotting those numbers (current at 0.2 on x, Current at 1000F on Y) would (hopefully) shows linearity - which would be useful for determining target using only current measurement.
Could you measure current at say 0.2 seconds and the point you measure 1000F for a few different cases? I suspect plotting those numbers (current at 0.2 on x, Current at 1000F on Y) would (hopefully) shows linearity - which would be useful for determining target using only current measurement.
Recording current at 0.2s and at 1000F for a bunch of different cases doesn't prove a linear ramp of current, and more importantly, case temp between start and end. It would merely show consistency or not at two points in time. You need to record current and temp at as many points during the time from room temp to 1000F, and beyond, as you can (and likely do a little smoothing to iron out measurement variability).
@oliverpsmile what temp sensor are you using?
@oliverpsmile what temp sensor are you using?

Discover Arduino Flame Sensor for Fire Detection!
Uncover the capabilities of the Arduino flame sensor—your essential tool for fire-fighting and soccer robots. Detects light wavelengths 760-1100nm.
Thanks. But how do these measure temp when no 'flame' is present? That is, from a case sitting at room temperature up to glow. I get how these can be used to detect the glow of a case close to melting but they'd not seem to offer much use in measuring temperature before that point. Confused...
The brass must glow in order to be annealed. The moment the brass starts glowing you can trust the sensor. This is an infrared sensor and I believe it reads the heat waves without glowing But this is totally irrelevant as far brass annealing is concern.Thanks. But how do these measure temp when no 'flame' is present? That is, from a case sitting at room temperature up to glow. I get how these can be used to detect the glow of a case close to melting but they'd not seem to offer much use in measuring temperature before that point. Confused...
Ok I now understand the reluctance to post the temperature data and why you're looking for 1000F Tempilaq. You still need to calibrate the data from the IR sensor to a known reference as it isn't outputting temperature. It just churns out an uncalibrated data level. Of course, there's a little glow, a bit more glow, medium glow and 'holy crap it's glowing' (and all points in between). Thankfully most of these one can see with the naked eye. (See below for more on this.) And if one isn't trying to automate across widely varying brass (I use the same brand of brass and turn my brass necks to reduce case to case variance and so this doesn't apply to me and I suspect many others) a point at which samples of a brass batch glow can be determined directly by visual observation. Of course one needs to know if it's "glowing enough" (and for long enough) to reach a hypothetical target that in turn has to be proven by actual empirical hardness testing - but one step at a time. And so you're back trying to calibrate your sensor - or eye - back to a known target. The cool thing about Tempilaq (choose your temp between 300F and 1500F) is that it provides a visible temp indicator.
The advantage of your sensor is that once you know the sensor data level that corresponds to, for example, 1000F (or whatever) there's no further need to run test cases which saves a bit of work. Cool if its reliable and works within the temp range you need.
Either way you are left with a leap of faith that your target (temp and duration) yields the annealing result.
When one examines the cost of devices that can read, with accuracy and reliability, temperature across the scale needed here (see devices by the likes of Raytek) it's not surprising that AMP stuck with a methodology reliant on measuring current. I wonder to what extent they bothered to see how case temp tracked over the time from room temp to Curie or whether they simply modeled (through heavy sample analysis) case hardness from start to Curie. I wonder if this change was linear or much more complicated... Without conducting our own hardness testing we may never know.
Back to just temp. The cool thing about looking at a glowing object is that the temperature of the glowing object can be determined by its color and it virtually doesn't matter what the emitter is (brass, steel or whatever). See the chart below. Yes, to reach 1kF it "the brass must glow" but only barely so. 1000F is a glow that barely makes it into the visible spectrum - it's just detectable with the naked eye albeit just as well for us that it skimps in. But it's a very faint dull red (in a dark room). If you are seeing anything in, for example, what the chart below calls 'bright cherry' you're waaay over 1kF.

The advantage of your sensor is that once you know the sensor data level that corresponds to, for example, 1000F (or whatever) there's no further need to run test cases which saves a bit of work. Cool if its reliable and works within the temp range you need.
Either way you are left with a leap of faith that your target (temp and duration) yields the annealing result.
When one examines the cost of devices that can read, with accuracy and reliability, temperature across the scale needed here (see devices by the likes of Raytek) it's not surprising that AMP stuck with a methodology reliant on measuring current. I wonder to what extent they bothered to see how case temp tracked over the time from room temp to Curie or whether they simply modeled (through heavy sample analysis) case hardness from start to Curie. I wonder if this change was linear or much more complicated... Without conducting our own hardness testing we may never know.
Back to just temp. The cool thing about looking at a glowing object is that the temperature of the glowing object can be determined by its color and it virtually doesn't matter what the emitter is (brass, steel or whatever). See the chart below. Yes, to reach 1kF it "the brass must glow" but only barely so. 1000F is a glow that barely makes it into the visible spectrum - it's just detectable with the naked eye albeit just as well for us that it skimps in. But it's a very faint dull red (in a dark room). If you are seeing anything in, for example, what the chart below calls 'bright cherry' you're waaay over 1kF.
Last edited:
One additional thought. The wavelength of light in the visible spectrum is between approximately 380 and 750nm, 750 being at that barely discernible "faint red" end of the spectrum. But something glowing very faintly red is emitting far more than the light we see. It is giving off much more radiation at far longer wavelengths that we don't see but rather feel in the form of heat. I believe Wein's displacement law can be used to determine this peak wavelength emission.

 en.wikipedia.org
en.wikipedia.org
A sensor trying to detect fire will have an operational spectral bandwidth: "760nm to 1100n" in the case of your sensor. This sensor range needs to 'conform' with the ability to detect that faint, dull red that's in line with your target 1kF. A bright fire is by definition much hotter than a dull red cartridge case. It's peak radiation will be at much shorter wavelengths.
To apply Wein's displacement law to 1kF (= 810K) I think (note italic emphasis) we can simply drop in 810K into the formaula and get a peak radiation wavelength of circa 3,573nm. In contrast, the color temp of a candle flame is about 1800K with a peak radiation in the 1610nm wavelength.
In short, I think you need to be wary of such detectors and their operational ranges and hence their suitability for detecting reliably what are, in the scheme of fire detectors, relatively cool temperatures.If nothing else dial up their sensitivity to or near the max.
**** You could try calibrating your sensor using an old stove heating element (or similar) to which you could attach directly a suitable thermometer, inching the dial slowly to achieve your target temp. ****

Wien's displacement law - Wikipedia
A sensor trying to detect fire will have an operational spectral bandwidth: "760nm to 1100n" in the case of your sensor. This sensor range needs to 'conform' with the ability to detect that faint, dull red that's in line with your target 1kF. A bright fire is by definition much hotter than a dull red cartridge case. It's peak radiation will be at much shorter wavelengths.
To apply Wein's displacement law to 1kF (= 810K) I think (note italic emphasis) we can simply drop in 810K into the formaula and get a peak radiation wavelength of circa 3,573nm. In contrast, the color temp of a candle flame is about 1800K with a peak radiation in the 1610nm wavelength.
In short, I think you need to be wary of such detectors and their operational ranges and hence their suitability for detecting reliably what are, in the scheme of fire detectors, relatively cool temperatures.If nothing else dial up their sensitivity to or near the max.
**** You could try calibrating your sensor using an old stove heating element (or similar) to which you could attach directly a suitable thermometer, inching the dial slowly to achieve your target temp. ****
I'll try again. I'm not proving linear ramp of current or trying to. I have discovered linear relationship between current at 0.2 and current when a case starts to glow across different (as in caliber, head stamp) cases. Did you see the post I linked to? Example. Measure current for a 308 norma case at 0.2s (or any other time before glow) Measure current for 308 niorma case when it starts to glow (use any color from your metals temperature chart). Do the same for 223, 6.5 whatever.. 338, 375 cheytac..Recording current at 0.2s and at 1000F for a bunch of different cases doesn't prove a linear ramp of current, and more importantly, case temp between start and end. It would merely show consistency or not at two points in time. You need to record current and temp at as many points during the time from room temp to 1000F, and beyond, as you can (and likely do a little smoothing to iron out measurement variability).
Make a graph. Current for 0.2s on x-axis, current for your chosen glow color on the Y.
I made the graph in the post, where I described it.
So now I'm predicting current when ANY case starts to glow without having to destroy it. I'll just start an annealing cycle - measure current along the way, at 0.2s I know what the current will be when case starts to glow. If I know the temperature when the case starts to glow, I can provide a "hold time", where I translate time into energy (current will still rise - pretty much linear, which others already discovered), and since I measure current continuously, I will know how current increases with time, and I'll pretty much just need to add another x ampoere seconds which I assume would be hold time times current at target temp.
I'll attach the graph again - current at t=0.2 and current at (visually observed) glow. Different cases (head stamp, caliber)
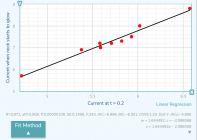
Last edited:
All credits to them that inspired all of our participants to build a "better mouse trap".It's really nice to see what this thread has become. From the basic GinaErik annealer, to discovering the current current drops off the second the brass melts (which really makes sense once you think about it and the eddy currents) to what's going on now.
Yes I understand what you are doing now.I have discovered linear relationship between current at 0.2 and current when a case starts to glow across different (as in caliber, head stamp) cases.
What I was hoping we could see is how temp behaves as current (for a given case) rises linearly over time. Unfortunately we haven't been able to observe that behavior because no one yet has been able to measure temperature. Oh well. Like I said, I wonder if AMP even bothered to and instead just relied on examining time as % of time to destruction (identified by observing current) vs impact on hardness.
So....you've still traded one calibration issue for another. Way back when, people used to anneal "to a faint glow in a darkened room" (or similar). They then tried to use Tempilaq as a slightly more impartial yardstick. But of course the darkening of Tempilaq is open to great interpretation. What change in color represents the indicator temp being reached and just where should it be placed. I'm not sure where the choice of a 750F indicator came from but I doubt it was because people necessarily believed 750F was the "right temp". That would certainly have conflicted with the "faint glow in a dark room" temp which is circa 950/1000F. I suspect, as I've said before, placing the 750F indicator lower down the case wall was likely felt adequate because it was obvious everything above it got much hotter. Maybe it was just that 750F was more readily available and people were forced to use it.
So now with a flame sensor you're still left wondering if you got the relevant area of the case to a sufficiently high temp but not too high that the brass becomes putty like. From personal experience I know it is VERY easy to over-anneal and end up with case necks and shoulders that won't stand up to a solid squeeze between thumb and forefinger let alone resizing in a press. As I noted in my prior post you might be able to do a lot better than merely looking for that faint, barely visible, dull red glow, assuming the sensor is reliable at low temps such as 1000F (assuming that's your presumed target) which itself could be tested also. The inability to touch the object being worked and the very short time periods are just a function of annealing cartridge brass. You can "calibrate" your sensor elsewhere under much more friendly conditions. I mentioned an old-fashioned heating element but one can think of other options. You just need to be able to slowly control its temperature (very finely tuned 'power' dial or such a dial coupled with a variac on the mains might provide really fine control) and have an accurate spot thermometer. Using a situation where the thermometer can touch the element heating makes things a lot easier and cheaper. Even my modestly priced Brymen digital multimeter can measure temperature between -58F and 1832F with an accuracy of 0.3%+3F, plenty good enough for these purposes.
Brymen BM869s multimeter
The Brymen BM869s is a high performance multimeter that has no problem matching up to the expensive Fluke meters when it comes down to display, possibilities and accuracy.
Once you've got your annealer finely and accurately tuned to 1000F it will be time to see if cases actually softened to the desired amount. But for that you will need an accurate hardness tester...
Exactly - but maybe there are cheap and easy workarounds.So....you've still traded one calibration issue for another. Way back when, people used to anneal "to a faint glow in a darkened room" (or similar). They then tried to use Tempilaq as a slightly more impartial yardstick. But of course the d
Once you've got your annealer finely and accurately tuned to 1000F it will be time to see if cases actually softened to the desired amount. But for that you will need an accurate hardness tester...
1.
Maybe I'm lucky that by adjusting the constant for the function found for the unknown target temperature (moving the curve up or down the y-axis, effectively changing target for another temperature/cu) I can find a sweet spot for another unknown temperature using a fixed "hold time/energy" . It requires the coefficient of the function to not change. The sweet spot would be determined with either hardness testing or "Annealing ladder test". A "cheap" portable hardness tester is used in this video
To get an idea of whether or not the curves for different target currents (temperatures) are parallell maybe the IR sensors adjusted for different sensitivity are good enough, and the IR sensor guys could be helpful providing the data?
2. Using the target current (unknown temperature) predicted by the function as is and adjusting the hold time (energy) until wanted hardness is achieved. (hopefully finding a relationship between target current and hold time/energy)
Last edited:
Similar threads
- Replies
- 74
- Views
- 47,365
- Replies
- 0
- Views
- 1,576
Upgrades & Donations
This Forum's expenses are primarily paid by member contributions. You can upgrade your Forum membership in seconds. Gold and Silver members get unlimited FREE classifieds for one year. Gold members can upload custom avatars.

Click Upgrade Membership Button ABOVE to get Gold or Silver Status.
You can also donate any amount, large or small, with the button below. Include your Forum Name in the PayPal Notes field.
To DONATE by CHECK, or make a recurring donation, CLICK HERE to learn how.

Click Upgrade Membership Button ABOVE to get Gold or Silver Status.
You can also donate any amount, large or small, with the button below. Include your Forum Name in the PayPal Notes field.
To DONATE by CHECK, or make a recurring donation, CLICK HERE to learn how.









