Thanks for posting this information. Unfortunately there is a lot of "data" and "testing" that has been done that forcibly modifies the moisture content (powder does not have humidity) of powder and its effect on velocity. There is little to no test data that shows how powder moisture changes with time and ambient humidity. Powder takes time to change its moisture level in relation to air because the transfer mechanism occurs only on the surface and the moisture is contained throughout the kernel. In normal storage and loading most of the powder resides in a packed column that does not allow free movement of air.
When poured kernels are exposed to free air for only fractions of a second the change in moisture is minimal and at the surface. Powder in a sealed but non hermetically sealed container does not magically come to equilibrium with the air outside the container. Even with changes in barometric pressure and temperature very little air is exchanged compared to the air in the container. The HDPE container itself essentially allows no movement of air or moisture (it does, but the amount is extremely small). The physics and chemistry involved is complex. Suffice it to say that the effects of humidity variations in normal storage and proper handling of powder can be measured in days or weeks and years, not minutes or hours.
The amount of moisture in the air in an 8LB powder container is extremely small compared to the moisture in the powder. Air at 70 degrees and 99% humidity in an 8lb container of has about 0.00018 lbs of water. One pound of powder of at 1% moisture has 0.01lb of moisture (water). If the powder were to absorb all of the moisture in the air (which it would not) in that container it would only increase the moisture content of the powder to 1.018%. If the powder had been in equilibrium at 70 degrees F and 50% Relative Humidity it would have absorbed only 0.00009 lbs and the powder moisture would have increased to only 1.009%.
There are places where longterm storage in unconditioned spaces can have a very long term effect. One such case is Las Vegas.
View attachment 1608370View attachment 1608370View attachment 1608370
If stored in this environment in a non hermetically sealed container the powder would ultimately acclimate to equilibrium at approximately 31% but the process would take years in an unopened container.
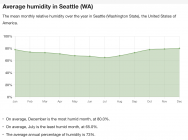
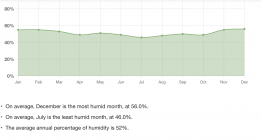
Another location of interest might be Seattle.
View attachment 1608371
Here the powder would ultimately reach equilibrium with about 73%.
So what about Denver CO?
View attachment 1608384
This humidity data is from
https://weather-and-climate.com/

 chronoplotter.com
chronoplotter.com

 chronoplotter.com
chronoplotter.com